J.P. Morgan’s Annual Healthcare Conference is the industry’s largest, most informative healthcare investment symposium. Attracting industry leaders, emerging fast-growth companies, and technology creators from around the world, life science corporations share their latest advances, leading research, and best practices—as well as their intentions to scale their digital health solutions—to the masses in hopes of securing capital.
This year, the Conference covered developments ranging from digital health and the integration of artificial intelligence (AI) to deep learning, gene editing, bioengineering, implantable technology, medical funding, personalized care, service automation, and much more. Needless to say, there were thousands of invaluable talking points made and thought-provoking insights shared over the course of the four-day event.
Using the AlphaSense platform, we reviewed the assortment of transcripts emerging from the Conference to uncover the key trends and developments shaping the healthcare space in 2024 and beyond.
GLP-1 Agonists and Precision Medicines
It’s no surprise that GLP-1 agonists—a class of medications that can help manage Type 2 diabetes and obesity—took center stage at the Conference. Since the rise of Ozempic and Wegovy last year, GLP-1 agonist patenting and production has seen major activity. To date, the biopharma landscape already boasts over ten FDA-approved medications dedicated to weight loss, all contributing to the expanding array of options for individuals seeking effective weight management solutions.
The market leader, Novo Nordisk, is already strategizing the next phases for its obesity drug, Wegovy. The company aims to explore different dosages and alternative methods of drug delivery, moving away from the current injectable option. Overall, there are expectations of a doubling in GLP-1 production capacity this year.
“If you look at our growth in ’23, Q3 year-to-date, we grew Ozempic by 50%. We grew Wegovy by close to 500% obviously, from a low base. And Ozempic is the best-selling diabetes product in the world today. So the fact that we grew that by 50% talks a bit to the magnitude of the capacity expansions we are doing, and I’m very comfortable looking into ’24 and specifically for Wegovy and the U.S. that we can add significant additional volumes also in ’24.”
“So it’s a situation where we gradually scale up manufacturing, we have lines coming in. So there’s not like a one triggering event. Unlocking this it’s a continued journey of building capacity for the years to come, but a significant additional capacity for ’24 for sure.”
– Lars Fruergaard Jorgensen | President, CEO & Member of Management Board of Novo Nordisk
Precision medicine—medical care designed to optimize efficiency or therapeutic benefit for particular groups of patients, especially by using genetic or molecular profiling—was also a major topic of discussion. Voyager Therapeutics is gearing up to submit at least four investigational new drug applications to the FDA within the next two years. Additionally, the Norwegian company Nykode anticipates progressing its human papillomavirus (HPV)16-targeted vaccine into clinical trials.
“We plan to file an IND with our lead program, VY-TAU01 in a few months. That should let us proceed to a Phase Ia Single Ascending Dose study this year. And then next year, we’ve initiated a Phase Ib trial, where we plan to employ tau PET imaging to hopefully obtain a proof of concept. And what we’re trying to do is to see whether the antibody blocks the spread of tau in humans with Alzheimer’s disease.”
“And we expect the key readout from that to occur in H2 of 2026. So that’s why it was important to extend the runway into 2027. In addition to that program, we have several gene therapy programs entering—we expect to enter into the clinic in the 2025 time frame. So our own wholly-owned SOD1 ALS program, we expect to enter the clinic in mid-2025 and Neurocrine has already said that they expect to initiate 2 gene therapy programs in the same year.”
– Alfred W. Sandrock | President, CEO & Director of Voyager Therapeutics
AI’s Takeover in Medicine
According to an industry report, AI in drug discovery is set to reach $5 billion in the next four years, reaching an evaluation of $4.9 billion by 2028 from USD 0.9 billion in 2023 (a CAGR of 40.2% during the forecast period). Already, AI is playing a transformative role in R&D due to its ability to cross-reference published scientific literature with diverse information sources, such as clinical trial data, conference abstracts, public databases, and unpublished information.
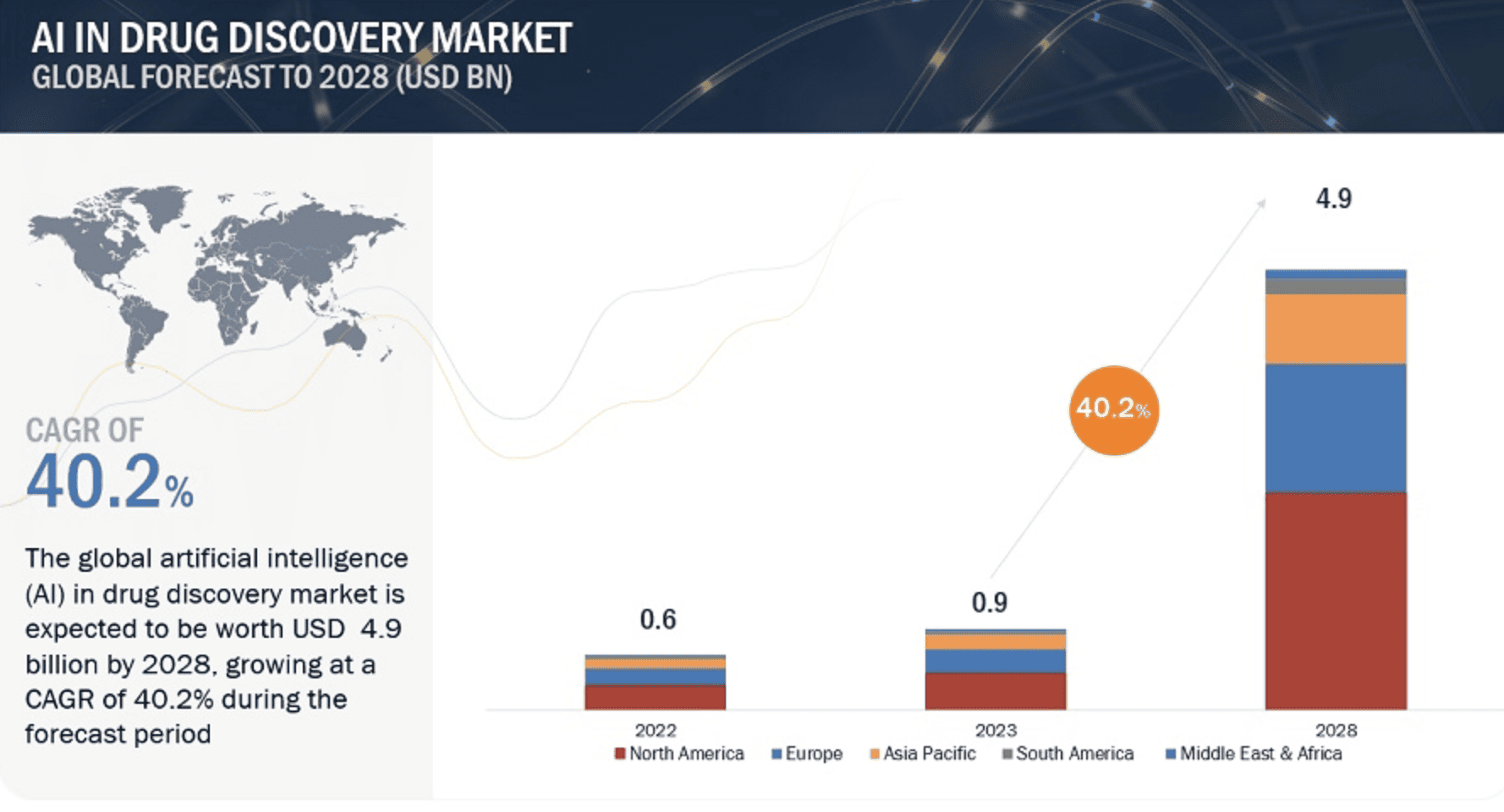
This approach facilitates swift identification of new molecules and promising therapies, ultimately yielding new candidate medicines in a matter of months, as opposed to traditional timelines spanning years. The bottom line: AI will significantly enhance R&D productivity and save millions of dollars for drug developers.
“What we did here using our AI engine called dAIsY to try and see how we could create a next-generation COVID antibody that would be really variant-proof. And what we have been successfully showing now is that VIR-7229 in vitro is active against the next generation of variants that are circulating today, such as, for example, JN.1, which is now 60% of the variants that are circulating in the United States.”
– Marianne De Backer | CEO & Director of Vir Biotechnology
“Deepcell’s REM-I is a new platform for cell imaging. It does cell sorting, imaging and high dimensional analysis all in 1 instrument, and they’re creating a new field called morpholomics. This is a video simulating a melanoma cell, physically transforming to a functional state that gives it the ability to transmit to other tissues, otherwise known as metastasis. This is highly significant insights and information for both the research community and the clinical community. It’s the next generation of flow cytometry and digital pathology put into 1 instrument.”
“And by using their generative AI algorithms, they have one called the human foundation model, they can do that real-time analysis of characterization of those cells right on the instrument using NVIDIA GPUs and our AI stack. This platform is inventing a new omics field called morpholomics.”
– Kimberly Powell | Vice President of Healthcare at Nvidia
The Rise of CNS Therapeutics
An area of healthcare that’s recently gained traction at the Conference is central nervous system (CNS) disorders, which generated significant conversation with biotech companies like Neurocrine Biosciences, Intra-Cellular Therapies, and Anavex Life Sciences showcasing their latest treatments.
This renewed interest in rare disease therapeutics can be attributed to the U.S. Food and Drug Administration (FDA). Last year, over half of the approved drugs obtained the FDA’s recognition through an orphan-drug designation, specifically intended for the treatment of rare disorders. Further, Leqembi’s FDA approval for the treatment of Alzheimer’s disease emerged as a standout accomplishment in the field, setting the stage for subsequent successes in the industry last year.
“What we’ve learned from neuro-oncology is that we can actually change the course of the disease, and we believe that with CNS diseases, we can be curative by the end of this decade. And our goal is to build the leading neuroscience company in the world. And to do that, you need to cure diseases. So as I said, we have this large pipeline that we have. We have 14 compounds in clinical development right now, in 17 different indications.”
– Kevin C. Gorman | CEO & Director of Neurocrine Biosciences
“Our commitment is that cutting-edge CNS treatments are available to an adverse patient population, ultimately enhancing the effectiveness and reach of healthcare systems. We believe that this focus on accessibility and sustainability represents an alternative and attractive opportunity in supporting innovative solutions for global healthcare challenges. And with our scientific advancement, we have gotten in very interesting indications, which have high unmet need, which includes Alzheimer’s Disease, Parkinson’s Disease Dementia, Parkinson’s Disease and Rett Syndrome.”
– Christopher U. Missling | President, CEO, Secretary & Director of Anavex Life Sciences
Staying Ahead of Developments in the Healthcare Industry
Scouting out new healthcare investment opportunities before your competition requires a cutting-edge research tool.
Want to dig even deeper into the top takeaways from J.P. Morgan’s 42nd Annual Healthcare Conference? Join our webinar, Top Takeaways: J.P. Morgan’s 42nd Annual Healthcare Conference, to hear more on the trends, deals, and drug developments shaping the future of the healthcare industry. In this roundtable with esteemed reporters from STAT and Endpoints News and Sara Mallatt Stahl, Director of Healthcare Research at AlphaSense, they will offer an insider’s view and share key insights from the conference.
Cut out the roadblocks that come from researching across disparate resources, and maximize your efficiency and effectiveness with an all-in-one market intelligence and search platform. With advanced AI search technology, AlphaSense helps you not just stay ahead but take the lead in your industry. Sign up for your free trial today.